By Michael A Grima (B.Sc. Hons I, GradCertVetStud)
A protozoal disease associated with the formation of small white spots/cysts on the gills and skin of marine and freshwater fish species. White spot disease, in the context of salt and freshwater environments, is not caused by the same species of protozoan, however, clinical signs of infection (symptoms) and pathogenesis (disease development) in both contexts is similar.
Causes:
-
Freshwater pathogen - Ichthyophthirius multifiliis.
-
Marine pathogen - Cryptocaryon irritans.
Diagnosis:
-
Scrapings of gill and/or skin tissue or body slime associated with cysts can be mixed with a drop of aquarium water and mounted on a glass microscope slide and then cover-slipped to be viewed microscopically for the identification of the protozoal parasites. Magnification of 100X – 400X is sufficient for optimal diagnosis.
-
Ichthyophthirius multifiliis (freshwater “Ich”) – in its mature cystic stage called the “Trophont” is characterised as a large, dark and spherical ciliated (surrounded by rows of fine beating hairs) protozoan with a characteristic “horseshoe” nucleus and tumbling motion when immersed in water. Immature “Tomites” are smaller, translucent and move more rapidly than mature “Trophonts”.
-
Crytopcaryon irritans (saltwater “Ich”) – identification of the “Trophont” from skin, fin or gill tissue is the best means of diagnosis. The organisms appear opaque and are spherical or pear shaped and ciliated. The “Trophonts” exhibit their characteristic “rolling” motion.
Transmission:
-
The transmission of the infection usually results from high stocking densities of fish in aquariums.
-
Temperature is another important factor to consider in the transmission of this disease, particularly in the transmission of Cryptocaryon in the marine context. The higher the temperature, the increased the rate of lifecycle completion of the parasite.
-
Fish may carry the parasite in their body slime or may be mildly infected upon purchase (cysts may or may not be present). Once introduced into the aquarium, they transmit the parasite readily leading to infection of the weakest fish of the tank which can result in a widespread outbreak. It is therefore useful to have a quarantine aquarium set up for monitoring of new fish, prior to their introduction to the display aquarium.
-
Replication of “Ich” parasites is rapid and a thus a seemingly minor infestation in an understocked aquarium can quickly become a major issue in an aquarium that is stocked to maximal carrying capacity.
-
Fish infected with other pathogens; bacterial, fungal or with other protozoa, are commonly co-infected with “Ich” pathogens. These other infections may be incidental to the primary “Ich” infection.
| Parasitic Lifecycles: |
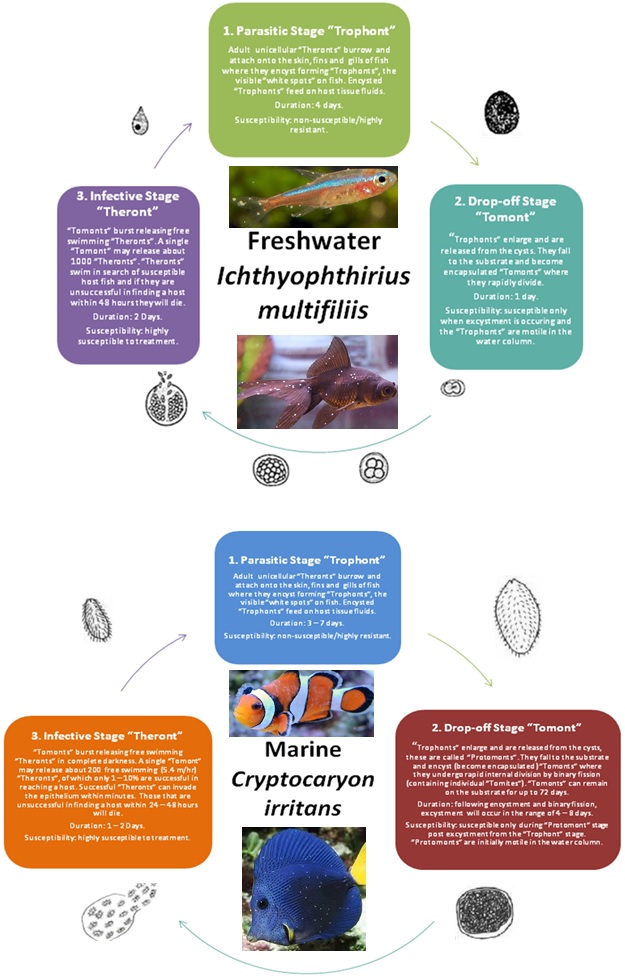 |
Symptoms:
-
Protozoal cystic nodules are visible to the naked eye, typically approximately 1mm in diameter. The presence of cysts has often been described as fish appearing to have had salt sprinkled on them.
-
During the cystic stage of the infection, fish may display behaviour associated with skin irritation. Fish are often seen rubbing against tank ornaments, rocks or gravel bed. Skin and gill irritation is usually associated with the cystic stage of the infection process and may not present during the free-swimming/”swarmer” stage called “Theronts”.
-
Degeneration of fins may be present in particularly severe infestations.
-
Microscopic examination of skin and gill scrapings often shows a thickening of the skin epithelium (tissue of the external body surface) and excess mucous production. Excessive mucous production may be appreciated with the naked eye in some severe cases.
-
The gills of fish are particularly susceptible to infection, due to their high surface area and increased vasoactive activity. Excessive mucous production in the gills usually occurs coupled with an increase rate of gill cell division (epithelial hyperplasia).
-
The gill operculum (hard bony flap protecting the gills) is often flared due to respiratory distress and fish may have an increased respiratory rate, characterised by frequent short shallow gulping episodes.
-
Severe and heavy infestations may result in lethargy leading to a decreased rate of respiration i.e. gulping may become shallow and sporadic.
-
Cysts that have burst often result in the formation of ulcers on the gills and skin and these tissue damaged areas often result in the predisposition of the fish to secondary infection with opportunistic fungal and bacterial pathogens.
Treatment and Prevention:
Quarantining &Temperature Adjustment
A quarantine period of at least 3–6 weeks at 24–27°C is effective. However longer periods (7–11 weeks) may be necessary. This gives time for encysted “Tomonts” to release infectious “Theronts” which will perish within 24–48 hours if they are unable to find a susceptible host. In a saltwater reef aquarium the quarantine procedure involves the removal/transfer of all “host” fish without altering temperature of the display tank. Already infected fish can be transferred to a well-aerated bare-bottomed quarantine tank at an elevated temperature (~ 27–28°C) and decreased salinity (1.015–1.020).Osmotic Challenge / Hyposalinity
(MARINE)
Hyposalinity: in a quarantine tank maintain water at 1.015 for 21 – 30 days. Gradual reduction of Salinity is important to prevent osmotic shock to fish; 1.005 per day until 1.015 is reached. This protocol should be used in conjunction with temperature reduction (see above).
(FRESHWATER)
Hypersalinity: 3 teaspoons (15 g) of pure sea salt per gallon (3.79 L) to achieve a specific gravity/salinity that is 1/10th that of natural seawater (1.020 – 1.029). Introduce saline solution gradually over a period of hours until the desired concentration is reached. Treat for a week before performing water changes to gradually lower the salinity post treatment period.
Copper Dosing
The minimum copper treatment period is 3–6 weeks. The administration of 0.15–0.20 mg/L free copper (ionic Cu2+) should be gradually obtained over a 2 – 3 day period (“breaking in” period for fish to reduce toxic effects of copper). The free copper concentration should be monitored daily for the duration of the treatment period to ensure the maintenance of the desired concentration range. The copper treatments usually provide copper in the form of copper sulfate pentahydrate which contains 25.5% “free”" and therefore active copper (Cu2+).
*IMPORTANT:
copper should only be used as a treatment in freshwater and marine FISH ONLY aquariums, as copper is highly toxic to invertebrates in both contexts*.
Formalin
Formalin is a 37 – 40% aqueous solution of formaldehyde. The safest method of using formalin as a treatment for protozoal infections both in the freshwater and marine context is in a well aerated quarantine aquarium. Formalin can be used in conjunction with osmotic challenge (see above) in a quarantine setting.
*IMPORTANT:
DO NOT use formalin in conjunction with elevated temperature as it readily strips the environment of dissolved oxygen as does significant temperature elevation. Ensure that the treatment environment is well aerated when using formalin.
DO NOT use if gill disease is suspected as formalin is a strong disinfecting poison and irritant leading to acute respiratory distress due to gill irritation.
DO NOT use where freshwater and marine invertebrates are present due to variable sensitivities of species to formalin. May have a mild antibacterial effect, it is therefore recommended that a bacterial supplement/booster be used post treatment to replenish potentially lost nitrifying bacterial load.
Formalin has an increased toxicity in soft water (acidic) environments, and therefore a lower dose should be used.*
Turn off UV sterilizers and aquarium lights for the duration of the treatment as formalin is light sensitive. As a bath, use at a rate of 0.15–0.25 mL/L for up to 1 hour and this can be repeated for 3 consecutive days, ensure extensive aeration during treatment. For prolonged immersion use at a rate of 0.015–0.025 mL/L, procedure may be repeated every 3–4 days however a partial water change is required between treatments.
Malachite Green
*IMPORTANT: may have a mild antibacterial effect, it is therefore recommended that a bacterial supplement/booster be used post treatment to replenish potentially lost nitrifying bacterial load. Freshwater and marine invertebrates have variable sensitivity to malachite green and thus should be avoided in display aquariums. Use in quarantine aquariums only*.
For a bath treatment, use at a rate of 1–2 mg/L for 30–60 minutes. This procedure can be repeated for a maximum of 4 consecutive days. For prolonged immersion treatment, use at a rate of 0.1–0.25 mg/L every 3 days for three treatments, before performing a partial water change.
Chloroquine
Usually used as a treatment for Velvet Disease (Amyloodinium sp.) but has been effective against “Ich” pathogens and is considered fairly stable.
Prolonged immersion of affected fish in 10 mg/L chloroquine diphosphate solution for 2 – 3 weeks. This procedure is best done in a quarantine tank with an elevated temperature and either hypersalinity (freshwater fish) or hyposalinity (marine fish).
UV Sterilisation
UV, based on freshwater “Ich” studies, is only effective against the infective free swimming “Theronts” not encysted “Tomonts”. These “Theronts” must physically pass through the UV steriliser to be destroyed. Any “Theronts” that do not, therefore have the potential to cause another outbreak.
The effectiveness of UV sterilisation in the marine “Ich” context has not been clearly elucidated. Both pathogens have common lifecycle stages, similar modes of transmission and pathogenesis but are genetically distinct from one another thus we cannot apply the effect of UV as seen in the freshwater “Ich” outbreaks to that which occurs in marine environments.

